Abstract
Introduction
Histone deacetylase (HDAC) inhibitors have single agent activity in various types of lymphoma. They restore antigen-specific immune recognition in B-cell lymphoma cells and modulate programmed cell death (PD)-1 expression on circulating T-lymphocytes. Pembrolizumab (PEM) is highly active in Hodgkin Lymphoma (HL) and demonstrates a 12-month PFS of 46% in patients with R/R HL. Preclinical studies have shown synergism of this combination in mouse models of various tumors. We present the interim efficacy analysis from the first stage of our phase II trial investigating the combination of the HDAC inhibitor Entinostat (ENT) and the PD-1-blocking antibody PEM in patients with R/R HL.
Methods
Patients with R/R HL received ENT 5-7 mg orally once weekly and PEM 200 mg intravenously once every three weeks. Prior use of anti-PD-1 or HDAC inhibitor was allowed if there had been clinical benefit. Tumor assessment was evaluated using the RECIL criteria. The primary endpoint is the 12-month progression-free survival (PFS).
Using a Simon two-stage minimax design to power the study, 21 patients were to be enrolled in the first stage with a 12-month PFS rate of 40% considered undesirable and 60% desirable.
Results
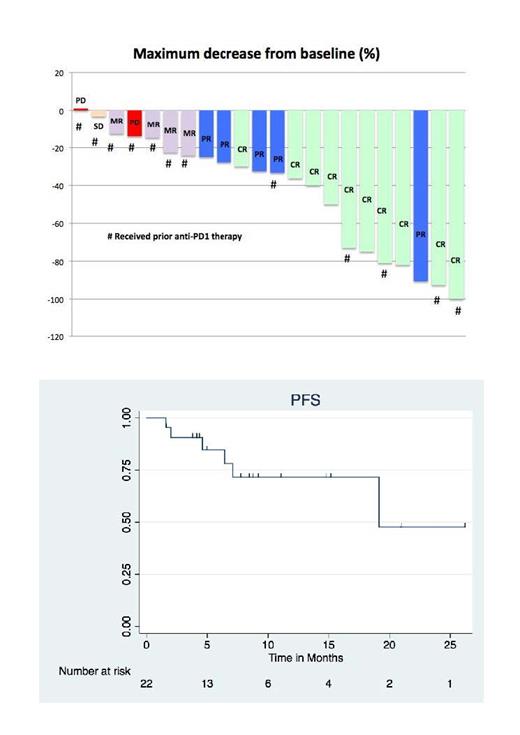
At time of data censoring on 7/24/21, 22 patients with HL have been enrolled. The median number of prior therapies was 5 (2-17). 7 (32%) were refractory to the most recent therapy, 13 (59%) had received autologous stem cell transplant (ASCT), 12 (55%) prior anti-PD1 antibody therapy, and 3 (14%) prior HDAC inhibitor therapy.
Out of 22 evaluable patients, the overall response rate (ORR) was 86% and the complete response (CR) rate was 45%. Responding patients included 9 with prior anti-PD-1 antibody and 3 with prior HDAC inhibitor therapy. With median duration of follow-up among survivors of 8.4 months (2-26), the 12-month PFS was 72% (44%-87%).
Reasons for treatment discontinuation included: progression (n=6), toxicities (n=5) consolidation with transplant or radiation (n=3), withdrawal of consent (n=3), and completion of study protocol (n=2). Severe toxicities resulting in study discontinuation were pleural effusions, pericarditis, pneumonitis and peripheral neuropathy. Out of the 22 total patients with HL enrolled in this study, 50% of patients had grade ≥3 (41%) and thrombocytopenia (32%). 41% exhibited grade ≥3 non-hematologic AEs, which included pericardial or pleural effusions (n=2, 9%), as well as fatigue, musculoskeletal pain, abdominal pain, pneumonitis, elevated lipase, hyperglycemia, and peripheral neuropathy. AEs that were potentially immune-related included hypothyroidism (n=2, 9%), elevated transaminases (n=4, 18%), diarrhea (n=3, 14%) and pneumonitis (n=2, 8%).
Conclusions
Interim results following stage I of this phase 2 trial demonstrates a 12-month PFS rate of 74%, meeting the primary endpoint of the study and justifying continued investigation of the combination of PEM and ENT. In this heavily pretreated patient population, responses were seen in the majority of patients despite prior exposure to anti-PD-1 agents.
Vardhana: Immunai: Membership on an entity's Board of Directors or advisory committees. Moskowitz: Bristol-Myers Squibb: Research Funding; Seattle Genetics: Consultancy, Research Funding; ADC Therapeutics: Research Funding; Beigene: Research Funding; Miragen: Research Funding; Janpix Ltd.: Consultancy; Merck: Consultancy, Research Funding; Imbrium Therapeutics L.P./Purdue: Consultancy; Takeda: Consultancy; Incyte: Research Funding. Joffe: AstraZeneca. Epizyme: Consultancy. Khan: Seattle Genetics: Research Funding. Kumar: Seattle Genetics: Research Funding; Astra Zeneca: Honoraria, Other: Advisory Board, Research Funding; Kite Pharmaceuticals: Other: advisory board , Research Funding; Pharmacyclics: Research Funding; Abbvie Pharmaceuticals: Research Funding; Celgene: Honoraria, Other: advisory board, Research Funding; Adaptive Biotechnologies, Celgene, Abbvie Pharmaceticals, Pharmacyclics, Seattle Genetics: Research Funding. Zelenetz: Gilead: Honoraria; Genentech/Roche: Honoraria, Research Funding; AstraZeneca: Honoraria; MorphoSys: Honoraria; Novartis: Honoraria; Pharmacyclics: Honoraria; Verastem: Honoraria; SecuraBio: Honoraria; BMS/Celgene/JUNO: Honoraria, Other; MethylGene: Research Funding; Abbvie: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; MEI Pharma: Honoraria, Research Funding; Janssen: Honoraria; Beigene: Honoraria, Other, Research Funding; Amgen: Honoraria; NCCN: Other; LFR: Other. Horwitz: Affimed: Research Funding; ADC Therapeutics, Affimed, Aileron, Celgene, Daiichi Sankyo, Forty Seven, Inc., Kyowa Hakko Kirin, Millennium /Takeda, Seattle Genetics, Trillium Therapeutics, and Verastem/SecuraBio.: Consultancy, Research Funding; Aileron: Research Funding; Acrotech Biopharma, Affimed, ADC Therapeutics, Astex, Merck, Portola Pharma, C4 Therapeutics, Celgene, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seattle Genetics, Shoreline Biosciences, Inc, Takeda, Trillium Th: Consultancy; Celgene: Research Funding; C4 Therapeutics: Consultancy; Crispr Therapeutics: Research Funding; Daiichi Sankyo: Research Funding; Forty Seven, Inc.: Research Funding; Kura Oncology: Consultancy; Kyowa Hakko Kirin: Consultancy, Research Funding; Millennium/Takeda: Research Funding; Myeloid Therapeutics: Consultancy; ONO Pharmaceuticals: Consultancy; Seattle Genetics: Consultancy, Research Funding; Secura Bio: Consultancy; Shoreline Biosciences, Inc.: Consultancy; Takeda: Consultancy; Trillium Therapeutics: Consultancy, Research Funding; Tubulis: Consultancy; Verastem/Securabio: Research Funding. Noy: Janssen: Consultancy, Honoraria; Epizyme: Consultancy; Morphosys: Consultancy; Rafael Parhma: Research Funding; Medscape: Consultancy; Targeted Oncology: Consultancy; Pharmacyclics: Consultancy, Research Funding. Batlevi: Memorial Sloan Kettering Cancer Center: Current Employment; Moderna: Current holder of individual stocks in a privately-held company; Viatris: Current holder of individual stocks in a privately-held company; BMS: Current holder of individual stocks in a privately-held company; Autolus: Research Funding; Pfizer: Current holder of individual stocks in a privately-held company; Dava Oncology: Honoraria; Bayer: Research Funding; Medscape: Honoraria; ADC Therapeutics: Consultancy; Regeneron: Current holder of individual stocks in a privately-held company; TouchIME: Honoraria; TG Therapeutics: Consultancy; Karyopharm: Consultancy; Seattle Genetics: Consultancy; Life Sciences: Consultancy; Kite Pharma: Consultancy; Juno/Celgene: Consultancy; GLG Pharma: Consultancy; Xynomic: Research Funding; Roche/Genentech: Research Funding; Novartis: Research Funding; Janssen: Research Funding; Epizyme: Research Funding. Matasar: Pharmacyclics: Honoraria, Research Funding; Juno Therapeutics: Consultancy; TG Therapeutics: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Rocket Medical: Consultancy, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Research Funding; Merck: Consultancy; Merck Sharp & Dohme: Current holder of individual stocks in a privately-held company; GlaxoSmithKline: Honoraria, Research Funding; ImmunoVaccine Technologies: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Memorial Sloan Kettering Cancer Center: Current Employment; IGM Biosciences: Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Teva: Consultancy; Janssen: Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy. Palomba: Nektar: Honoraria; Notch: Honoraria, Other: Stock; PCYC: Consultancy; Kite: Consultancy; Ceramedix: Honoraria; Novartis: Consultancy; Priothera: Honoraria; Juno: Patents & Royalties; Wolters Kluwer: Patents & Royalties; Lygenesis: Honoraria; Pluto: Honoraria; Rheos: Honoraria; BeiGene: Consultancy; Seres: Honoraria, Other: Stock, Patents & Royalties, Research Funding; WindMIL: Honoraria; Magenta: Honoraria. Lee: Intellisphere, LLC: Consultancy. Dogan: EUSA Pharma: Consultancy; Takeda: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Peer View: Honoraria; Seattle Genetics: Consultancy; Physicians' Education Resource: Honoraria. Salles: Abbvie: Consultancy, Honoraria; BMS/Celgene: Consultancy; Kite/Gilead: Consultancy; Ipsen: Consultancy; Janssen: Consultancy; Genmab: Consultancy; Takeda: Consultancy; Morphosys: Consultancy, Honoraria; Rapt: Consultancy; Genentech/Roche: Consultancy; Epizyme: Consultancy, Honoraria; Beigene: Consultancy; Debiopharm: Consultancy; Regeneron: Consultancy, Honoraria; Loxo: Consultancy; Miltneiy: Consultancy; Novartis: Consultancy; Incyte: Consultancy; Bayer: Honoraria; Velosbio: Consultancy; Allogene: Consultancy. Younes: AZ: Current Employment, Other: Senior Vice President, Global Head of Haematology (Early and Late Stage) Oncology R&D at AstraZeneca. von Keudell: Pharmacyclics: Consultancy, Honoraria; AbbVie: Research Funding; Janssen: Research Funding; Merck: Consultancy, Honoraria; Merck: Research Funding; Incyte: Consultancy, Honoraria; BMS: Research Funding.